Detect Imbalances in Vaginal Flora
Bacterial vaginosis (BV) is the most common cause of abnormal vaginal discharge in women of childbearing age.1 More than 50% of women diagnosed with BV experience recurrent symptoms within 12 months.2 The Aptima BV Assay is a Nucleic Acid Amplification Test (NAAT) that helps to detect one of the most common causes of vaginitis: bacterial vaginosis (based on algorithm using; Lactobacillus spp, Gardnerella vaginalis, Atopobium vaginae).3
Comprehensive, Accurate & Objective Results
The CE-marked Aptima BV Assay delivers reliable RT-TMA results for detection and quantitation of ribosomal RNA from bacteria associated with bacterial vaginosis.3
Accurate Diagnosis
An objective tool for the clinician to accurately diagnose and treat patients, with a higher sensitivity and specificity than clinician's diagnosis and in-clinic assessment.4
Greater Clarity
A clinical diagnosis that determines only a single pathogen is likely to be underdiagnosing infections that require different clinical management.5 Overlapping symptoms and co-infections make clinical diagnosis a challenge.6,7
Rapid Results
Time to first result in as little as 2 hrs 41 mins, with five additional results every five minutes.8
Improved Workflow
Benefit from superior workflow, reduced hands-on time and sample volume scalability from running assays on the fully automated Panther® System.9

Simplify & Scale the Future of Diagnostics
The Aptima BV Assay is part of the Hologic Molecular Scalable Solution, a portfolio combining a broad, high-performing assay menu with high-throughput automation. Designed to flexibly scale to meet your needs, from a single patient result to population-level screening.
Excellent Clinical Performance
Delivering a clear, objective result for Bacterial vaginosis.
Detection and quantitation
of rRNA from bacteria associated with bacterial vaginosis
Qualitative result
automatically determined by the assay software
Excellent sensitivity
and specificity from clinician and patient collected specimens
Overcome the Clinical Challenges of Accurate Diagnosis
Co-infections and overlapping symptoms make clinical diagnosis a challenge.6 Traditional methodologies are subjective and can impact treatment pathways.
Image source7
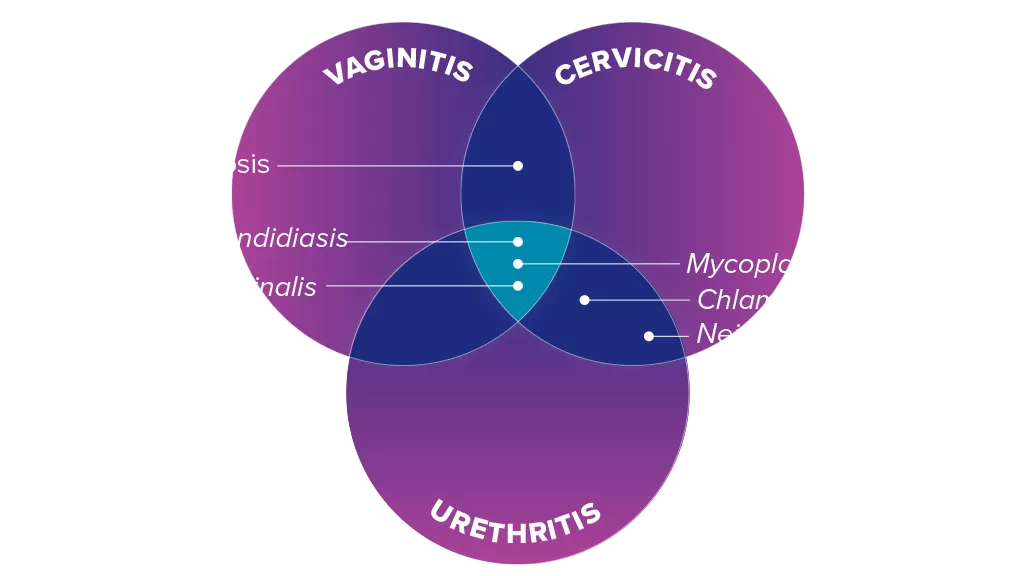
Test Together. Treat Differently.

Avoid Unnecessary Health Complications
Untreated BV infections can lead to an increased risk of complications including:
- Sexually transmitted infections (STIs) such as chlamydia, gonorrhoea, HSV and HIV3,10
- Pelvic inflammatory disease and cervicitis3,10
- Pregnancy-related concerns such as premature delivery, low birth weight and pregnancy loss3,10
Up to 30% of women diagnosed with BV were co-infected with Candida species11
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.
Insights
- Sherrard J, Wilson J, Donders G, et al. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. International Journal of STD & AIDS. 2018;29(13): 1258–1272
- Bradshaw C, Morton A, Hocking J, et al. High Recurrence Rates of Bacterial Vaginosis over the Course of 12 Months after Oral Metronidazole Therapy and Factors Associated with Recurrence. Journal of Infectious Disease. 2006 June; Volume 193, Issue 11: 1478–1486.
- Aptima BV Assay [Package Insert] AW-23712-001 Rev.001 San Diego, CA; Hologic, Inc., 2022.
- Schwebke JR, Taylor SN, Ackerman R, et.al. Clinical Validation of the Aptima Bacterial Vaginosis and Aptima Candida/Trichomonas Vaginitis Assays: Results from a Prospective Multicenter Clinical Study. J Clin Microbiol. 2020;58:e01643-19.
- Van der pol B, Daniel G, Kodsi S, et al. Molecular-based Testing for Sexually Transmitted Infections Using Samples Previously Collected for Vaginitis Diagnosis. Clin Infect Dis. 2019 Feb 1; 68(3): 375-381.
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291(11):1368–79.
- Adapted from Kent H. Epidemiology of Vaginitis. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 2):1168-76
- AW-26055-001 Rev. 001 Panther / Panther Fusion Operators Manual
- Ratnam S, Jang D, Gilchrist J et.al. Workflow and Maintenance Characteristics of Five Automated Laboratory Instruments for the Diagnosis of Sexually Transmitted Infections. J. Clin. Microbiol. 2014;52(7):2299-2304
- Aptima CV/TV Assay [package insert] AW-18812-001 Rev. 004., San Diego, CA; Hologic, Inc., 2021
- Sobel J, Subramanian C, Foxman B et al. Mixed Vaginitis—More Than Coinfection and With Therapeutic Implications. Current Infectious Disease Reports. 2013; 15:104–108.
Documents
Safety Data Sheets
Package Inserts
Related Products
1434
2797
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable