HPV Solution
A solution for Human papillomavirus (HPV) testing adapted to cervical screening programs, with a high-throughput, fully-automated workflow.

A Prognostic Approach to Eradicating Cervical Cancer
The Aptima® HPV Assay was introduced over a decade ago. Cross-sectional studies,1-6 longitudinal studies,7-9 and real-life screening program data10 have clearly demonstrated that HPV mRNA testing provides long-term protection, even after 10 years.7 The Aptima HPV Assay is a valuable screening assay to accelerate the elimination of cervical cancer worldwide.
The performance of the assay, combined with a high-throughput, fully-automated workflow and a complete sample traceability, makes the solution especially relevant for primary care national screening programmes.
Precision Targeting
The Aptima HPV assay targets mRNA within the 14 high-risk types of HPV, identifying active and clinically relevant infection. This targeted approach benefits patients and maximises resources.11
Improved Patient Experience
99.7% of cervical cancers are due to persistent HPV infection, making the presence of an active infection an excellent marker for further tests and potential treatment. Collecting just a single sample in our ThinPrep specimen collection device for subsequent cytological investigations avoids unnecessary stress and anxiety for women.12
Health Economic Savings
A model based on using the Aptima HPV assay as the foundation for UK primary screening (2.25 million female population), has estimated £15.4 million in savings for health authorities by preventing unnecessary treatment and follow-ups.13 The Health-Economics savings have also been confirmed for HPV primary screening programs in France, Spain and Canada.14-16
A Long-Term Commitment
We are market leaders in cervical cancer screening and women's health in the UK. We continue to invest to help implement, scale, and continuously improve screening programmes. We collaborate with organisations, NHS trusts and partners with a clear goal in mind: to eradicate cervical cancer.
Advancing the Early Detection of Cervical Cancer
1+ Billion17
ThinPrep vials used for global screening of cervical cancer
30 Countries17
supporting cervical cancer diagnosis and screening
100+ Million17
HPV tests sold globally
The Complete Solution for HPV Screening
Advances in molecular diagnostics have elevated the role of HPV detection in population screening. Improved sensitivity and accuracy enables10 countries to pursue a HPV primary strategy. Not only does this help to identify more women at risk of cervical cancer when compared to conventional cytology alone,18 but also alleviates staff shortages and workload pressures and is a more cost-efficient13-16 approach to screening. Explore the products in our solution here.

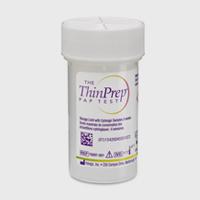
ThinPrep® PreserCyt Collection Vials
The ThinPrep Pap Test is a worldwide standard for cervical sample collection and preservation trusted by healthcare professionals around the world. More than 1 billion ThinPrep vials have been used globally for cervical cancer screening. Only one single patient sample is required for both cytology and molecular testing.19
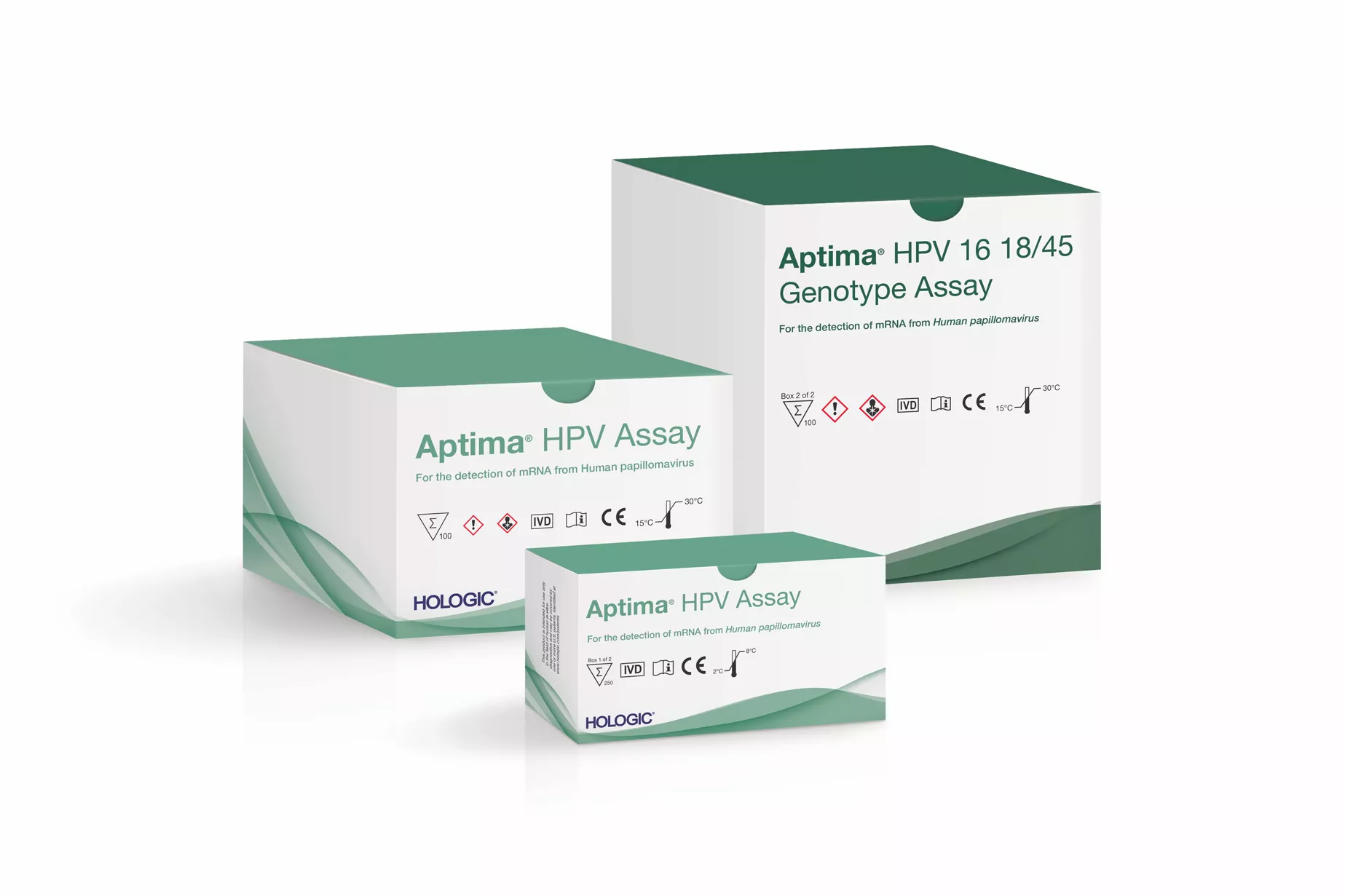
Aptima® HPV Assay
The Aptima HPV Assay is fully-validated for use in screening programs. It is one of the most validated HPV assays.20-24 The assay maximises the benefits of cervical cancer screening and minimises false-positive test results and potential for over-treatment. This allows clinicians to target the right patients for colposcopy which leads to more efficient screening programs and increased savings.11,25,26
Insights
Szarewski A, Ambroisine L, Cadman L et al. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):3033-3042.
Monsonego J, Hudgens MG, Zerat L, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid-based cytology in primary cervical cancer screening: the FASE study. Intl J Cancer. 2011 Aug;129(3):691-701.
Cuzick J, Cadman L, Mesher D, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013 Mar;108:908-913.
Iftner T, Becker S, Neis KJ, et al., Head-to-Head Comparison of the RNA-Based Aptima Human Papillomavirus (HPV) Assay and the DNA-Based Hybrid Capture 2 HPV Test in a Routine Screening Population of Women Aged 30 to 60 Years in Germany. J Clin Microbiol. 2015 Aug;53(8):2509-16.
Cook D, Smith LW, Law J, et al., Aptima HPV Assay versus Hybrid Capture® 2 HPV test for primary cervical cancer screening in the HPV FOCAL trial. J Clinical Virology 2017 Feb;87:23–29.
Haedicke J. , Iftner T. A review of the clinical performance of the Aptima HPV assay 2016 Mar;76 Suppl 1:S40-S48.
Strang THR, Gottschlich A, Cook D et al. Long-term cervical precancer outcomes after a negative DNA- or RNA-based human papillomavirus test result. Am J Obstet Gynecol. 2021 Nov;225(5):511.e1-511.e7.
Iftner et al., Longitudinal Clinical Performance of the RNA-Based Aptima Human Papillomavirus (AHPV) Assay in Comparison to the DNA-Based Hybrid Capture 2 HPV Test in Two Consecutive Screening Rounds with a 6-Year Interval in Germany, J Clin Microbiol. 2019 Jan 2;57(1):e01177-18. doi: 10.1128/JCM.01177-18.
Forslund O et al., HPV-mRNA and HPV-DNA detection in samples taken up to seven years before severe dysplasia of cervix uteri. Int J Cancer. 2019 Mar. 1;144(5):1073-1081. doi: 10.1002/ijc.31819.
Rebolj M, Cuschieri K, Mathews CS, et al. Extension of cervical screening intervals with primary human papillomavirus testing: observational study of English screening pilot data. BMJ 2022; 376:e068776.
Aptima HPV Assay [package insert] AW-22202 Rev 001. San Diego, CA: Hologic, Inc.; 2023
Walboomers et al Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):12-9.
Weston G, Dombrowski C, Harvey MJ, et al Use of the Aptima mRNA high-risk human papillomavirus (HR-HPV) assay compared to a DNA HR-HPV assay in the English cervical screening programme: a decision tree model based economic evaluation. BMJ Open. 2020 Mar 8;10(3):e031303.
Weston G, Dombrowski C, Steben M et al. A health economic model to estimate the costs and benefits of an mRNA vs DNA high-risk HPV assay in a hypothetical HPV primary screening algorithm in Ontario, Canada. Prev Med Rep. 2021 Jun 10;23:101448.
Dombrowski CA, Weston GM, Descamps PP et al. Health economic evaluation of an mRNA high-risk human papillomavirus (HR-HPV) assay versus a DNA HR-HPV assay for the proposed French cervical screening programme. Medicine (Baltimore). 2022 Jul 22;101(29):e29530.
Ibáñez R, Mareque M, Granados R et al. Comparative cost analysis of cervical cancer screening programme based on molecular detection of HPV in Spain. BMC Womens Health. 2021 Apr 26;21(1):178.
Data based on Hologic sales numbers since launch in 2012 to 31 Jan 2020.
Canfell K, Caruana M, Gebski V et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: Results of the Compass pilot randomised trial. PLoS Med. 2017 Sep 19;14(9):e1002388. doi: 10.1371/journal.pmed.1002388
ThinPrep® Pap Test PreservCyt Solution, Instructions for Use AW-22719-001 Rev 001
Zorzi M, Del Mistro A, Giorgi Rossi P et al. Risk of CIN2 or more severe lesions after negative HPV-mRNA E6/E7 overexpression assay and after negative HPV-DNA test: Concurrent cohorts with a 5-year follow-up. Int J Cancer. 2020 Jun 1;146(11):3114-3123.
Iftner T, Neis KJ, Castanon A et al. Longitudinal Clinical Performance of the RNA-Based Aptima Human Papillomavirus (AHPV) Assay in Comparison to the DNA-Based Hybrid Capture 2 HPV Test in Two Consecutive Screening Rounds with a 6-Year Interval in Germany. J Clin Microbiol. 2019 Jan 2;57(1):e01177-18. doi: 10.1128/JCM.01177-18.
Forslund O, Elfström M, Lamin H et al. HPV-mRNA and HPV-DNA detection in samples taken up to seven years before severe dysplasia of cervix uteri. Int J Cancer. 2019 Mar 1;144(5):1073-1081.
Strang THR, Gottschlich A, Cook DA et al. Long-term cervical precancer outcomes after a negative DNA- or RNA-based human papillomavirus test result. Am J Obstet Gynecol. 2021 Nov;225(5):511.e1-511.e7.
Rebolj: Rebolj M, Cuschieri K, Mathews CS et al. HPV pilot steering group. Extension of cervical screening intervals with primary human papillomavirus testing: observational study of English screening pilot data. BMJ. 2022 May 31;377:e068776.
Haedicke J. , Iftner T. A review of the clinical performance of the Aptima HPV assay 2016 Mar;76 Suppl 1:S40-S48.
Weston G, Dombrowski C, Harvey MJ, et al Use of the Aptima mRNA high-risk human papillomavirus (HR-HPV) assay compared to a DNA HR-HPV assay in the English cervical screening programme: a decision tree model based economic evaluation. BMJ Open. 2020 Mar 8;10(3):e031303
Panther®/Panther Fusion® System Operator's Manual AW-26055-001 Rev. 001 (EN)
Tomcat Instrument Operator's Manual, AW-26057-001 Rev001
Related Portfolios & Solutions

Molecular Scalable Solution
A scalable portfolio combining a broad, high performing assay menu with high throughput automation. Designed to flexibly scale to meet your needs, from a single patient result to population level screening.

Cervical Health
We pride ourselves on being champions of women’s health and global leaders in screening. From HPV to cytology, and now also AI based digital diagnostics, we offer a comprehensive and unique screening portfolio, from sample collection to diagnosis.
2797
Hologic BV, DA Vincilaan 5, 1930 Zaventem, Belgium
Notified Body number wherever applicable