FDA Market Authorisation for Genius™ Digital Diagnostics System
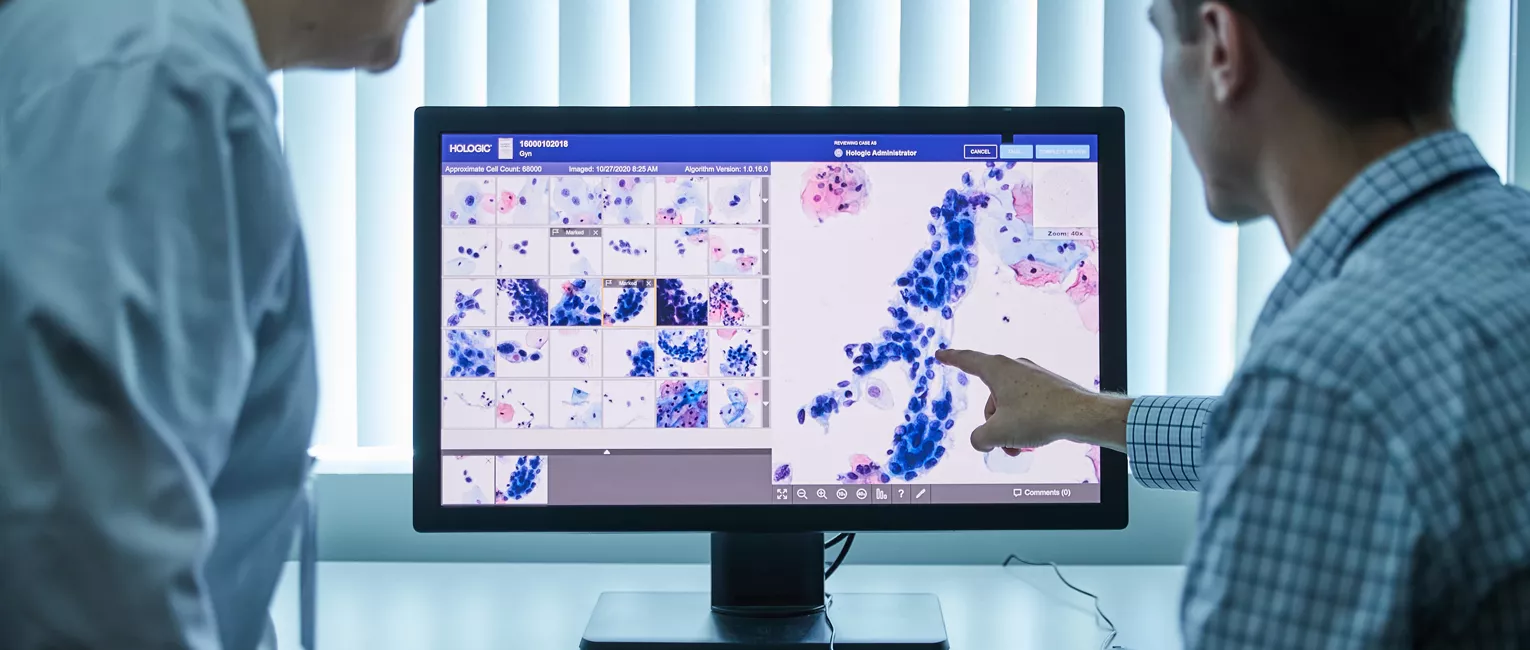
The system received its CE mark in 2021 and since then has been used to screen more than six million women for cervical cancer.*
The Genius Digital Diagnostics System is the next step in cervical cancer screening, empowering labs with a new artificial intelligence (AI) algorithm-based technology to help eradicate cervical cancer. This digital cytology system for cervical cancer screening can rapidly analyse cells on a digital image of the sample and present the most relevant images for review.1 This provides healthcare professionals with the support for early detection, improved workflow and enhance patient care.2
Globally, cervical cancer is the fourth most common cancer in women, with 604,000 new cases in 2020.3 When diagnosed, cervical cancer is one of the most successfully treatable forms of cancer, as long as it is detected early and managed effectively.4
Screenings for cervical cancer include a cytology test, where a sample is collected, and the cervical cells are sent to a lab where they are transferred to a glass slide. Traditionally, this glass slide has been reviewed under a microscope. With the Genius Digital Diagnostics System, the glass slides are digitally imaged and the AI algorithm is applied to pinpoint the cells that cytologists and pathologists should review.
Hologic technologies meet the needs of all commonly used national cervical cancer screening programs. The new system is part of our comprehensive cervical cancer screening portfolio.
More information on the FDA clearance can be found here.
To learn more, visit the product page for Genius Digital Diagnostics System.
2797
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable
* As per Hologic’s commercial data.
Genius Digital Diagnostics manual: AW-24823-001 Rev 001.
Ikenberg H, Lieder S, Ahr A, et al. Comparison of the Hologic Genius Digital Diagnostics system with the ThinPrep Imaging system - A retrospective assessment. Cancer Cytopathology 2023;doi: 10.1002/cncy.22695.
WHO. Cervical Cancer. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer#:~:text=Globally%2C%20cervical%20cancer%20is%20the,%2D%20and%20middle%2Dincome%20countries. Last accessed 5 December 2023.
WHO. Cervical Cancer. https://www.who.int/health-topics/cervical-cancer#tab=tab_1. Last accessed 5 February 2024.