Simplify & Scale Your Testing Capacity
Readiness for the respiratory viral season is a priority for every lab. As COVID-19 shifts from pandemic to endemic, accurate and timely detection and differentiation of viruses with flu-like symptoms will be crucial to guide patient care and assist infection control.
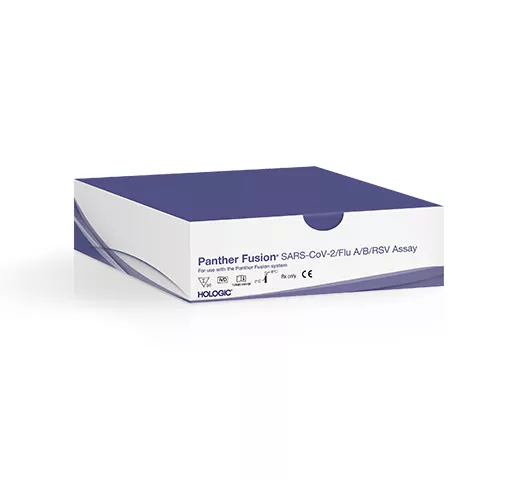
To help you simplify and scale testing capacity, the Hologic Molecular Scalable Solutions respiratory portfolio now offers multiplex capabilities, including for SARS-CoV-2. The new Panther® Fusion assay, covering Flu A, Flu B, RSV and SARS-CoV-2, enable high volume testing at scale of the most common respiratory viruses, helping you diagnose and deliver results sooner.1
Resilience to Cope With Seasonal Surges
We have built up our expertise in developing high-performing assays in the response to the unparalleled demands of SARS-CoV-2.
We help labs build first-class diagnostic capability and capacity, to meet seasonal demands and emerging global threats.
When demand peaks, you need a solution that you can trust and that deliver results.
Flexibility to do More With Less
We offer a fully automated, modular approach to syndromic respiratory testing, enabling you to target the right patient with the right test.
Our respiratory solution allows you to act quickly and at scale, with greater flexibility.
High throughput testing is available for the most common respiratory infections.
Boosting Workflow Efficiency & Clinical Insight
We continually invest in research and development to improve our portfolio to help meet your needs.
Our latest assay combines four targets in one high-performance assay, Flu A, Flu B, RSV and SARS-CoV-2. 1
The Panther® Fusion assay lets you test a single specimen from a patient who may have overlapping symptoms.1
Personalised Patient Testing2
Personalised patient testing enables accurate and timely diagnosis.
True random access and STAT applications offer workflow efficiency, allowing samples to be continuously loaded without the need to batch to perform multiple test orders.
This help you to prioritise urgent patients without impacting wider testing workflow.
Combining High Assay Performance with Automation
One sample and multiple testing capability depending on clinical needs gives you a modular approach for syndromic, respiratory testing.
2 Tiers
of automation with Panther and Panther Fusion providing scalable support.
3 Assays
for high-volume screening and differentiation of SARS-CoV-2.
13 Viruses
our family of assays targets multiple respiratory viruses.
1 New
assay covering Flu A, Flu B, RSV and SARS-CoV-2.
Explore the Respiratory Testing Solution
Aptima® SARS-CoV-2 Assay
Fully-automated, high-throughput assay for the detection of SARS-CoV-2.3 Meet the urgent need for high-throughput and fully-automated testing, delivering more than 1000 test results in 24 hours. Number of actual test results per day may vary based on individual lab practices and workflows.4

Panther® Scalable Solution
The Hologic high-throughput solution allows you to expand your testing menu while adding on flexibility, capacity and walkaway time. The Panther® System is the foundation of Panther Scalable Solutions. It lets you customise your testing by choosing from a broad assay menu and instrument add-ons, allowing an economical and scalable path to your growth.
Insights
Increase Efficiency & Reduce Costs
Accurate and timely diagnosis of the cause of respiratory tract infections has a number of benefits for patients and their communities, including:
- Reduces potential for further development of antimicrobial resistance due to antibiotics10
- Improves patient care and helps decrease costs10,11
- Provides valued information to public health authorities regarding which viruses are circulating in the community12
- Assists infection control personnel to provide appropriate measures to minimise nosocomial spread13

Panther Fusion SARS-CoV-2/Flu A/B/RSV assay [package insert]. AW-25328-001 Rev. 001. San Diego, CA: Hologic, Inc.; 2022
Panther®/Panther Fusion® System Operator's Manual AW-26055-001 Rev. 001 (EN)
Aptima SARS-CoV-2 assay [package insert]. AW-22752-001 Rev. 005. San Diego, CA: Hologic, Inc.; 2023
Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat Rev Microbiol. 2021 Mar;19(3):171-183. doi: 10.1038/s41579-020-00461-z. Epub 2020 Oct 14. PMID: 33057203; PMCID: PMC7556561.
Aptima SARS-CoV-2/Flu assay [package insert]. AW-22365-001 Rev. 004. San Diego, CA: Hologic, Inc.; 2023.
Panther Fusion Flu A/B/RSV assay [package insert]. AW-23081 Rev. 001. San Diego, CA: Hologic, Inc.; 2022.
Panther Fusion AdV/hMPV/RV assay [package insert]. AW-29005 Rev. 001. San Diego, CA: Hologic, Inc.; 2023.
Panther Fusion Paraflu assay [package insert]. AW-23708-001 Rev 001. San Diego, CA: Hologic, Inc.; 2023.
Panther Fusion Bordetella assay [package insert]. AW-18637-001, Rev. 001. San Diego, CA: Hologic, Inc.; 2018
Pinsky BA, Hayden RT. Cost-Effective Respiratory Virus Testing. J Clin Microbiol. 2019 Aug 26;57(9):e00373-19. doi: 10.1128/JCM.00373-19. PMID: 31142607; PMCID: PMC6711893.
https://www.health.vic. gov.au/health-advisories/testing-for-respiratory-pathogens Accessed August, 2022.
WHO-2019-nCoV-Surveillance-Guidance-2022.1-eng.pdf https://www.who.int/publications/i/item/WHO-2019-nCoV-SurveillanceGuidance-2022.2 Accessed October, 2022.
Lim RHF, Htun HL, Li AL, Guo H, Kyaw WM, Hein AA, Ang B, Chow A. Fending off Delta - Hospital measures to reduce nosocomial transmission of COVID-19. Int J Infect Dis. 2022 Apr;117:139-145. doi: 10.1016/j.ijid.2022.01.069. Epub 2022 Feb 4. PMID: 35124240; PMCID: PMC8813202.
Related Portfolios & Solutions

Molecular Scalable Solution
A scalable portfolio combining a broad, high performing assay menu with high throughput automation. Designed to flexibly scale to meet your needs, from a single patient result to population level screening.

Cervical Health
We pride ourselves on being champions of women’s health and global leaders in screening. From HPV to cytology, and now also AI based digital diagnostics, we offer a comprehensive and unique screening portfolio, from sample collection to diagnosis.
2797
Hologic BVBA Da Vincilaan 5 1930 Zaventem Belgium
Notified Body number wherever applicable
EC Rep details wherever applicable