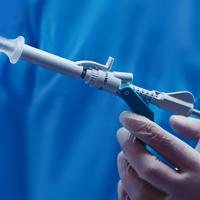
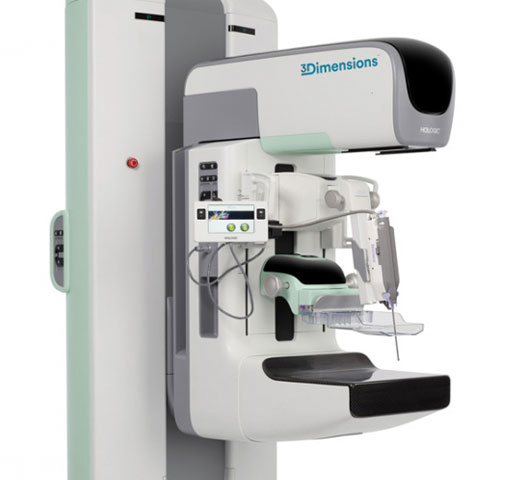
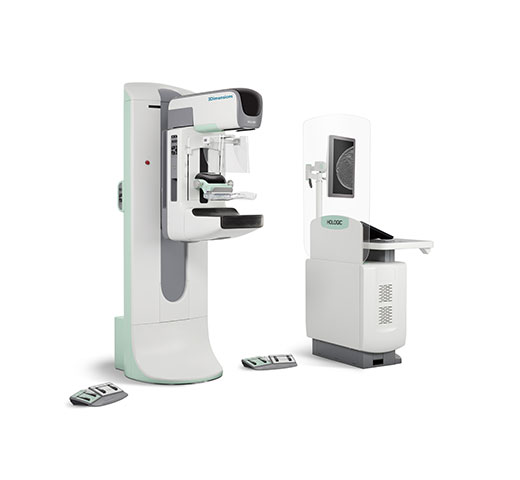
Breast Biopsy Site Markers
Distinct shapes with options for great long-term visibility. Designed so you can identify sites with confidence.

A Comprehensive Range
The Hologic range of biopsy site markers come in multiple shapes, gauges and lengths. The markers are compatible for use with x-ray, ultrasound and MRI guided biopsies. All markers come with an ergonomic and easy to use deployment device included.
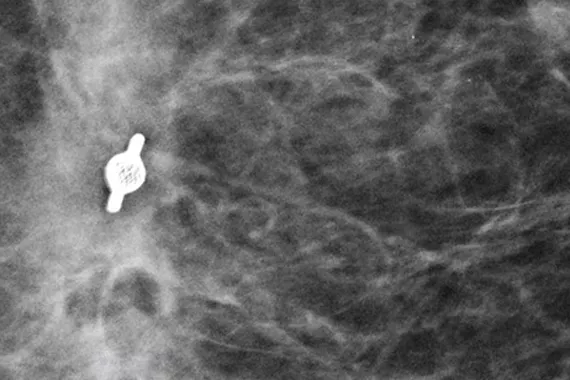
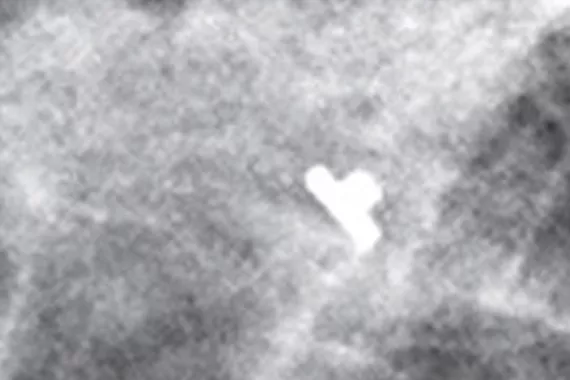
Tumark® Biopsy Site Markers
Intelligently designed to provide long-term visibility. All Tumark Markers are non-bioabsorbable, biocompatible permanent markers offering excellent visibility and are designed to minimise movement.1
SecurMark® Biopsy Site Markers
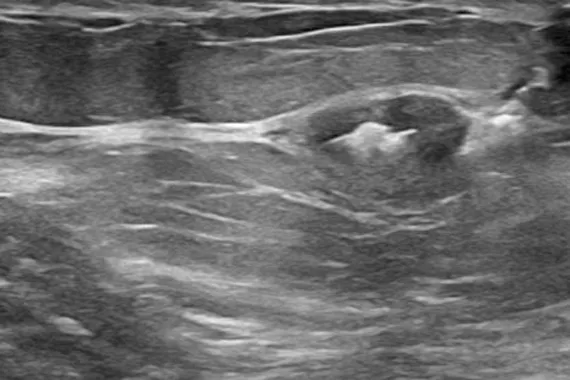
Designed to minimise movement,2 this marker is highly visible in ultrasound upon deployment and still highly visible at 6 weeks post-biopsy, crucial in case of future breast interventions.3 The marker consists of two pieces, a permanent marker and a bioabsorbable suture-like netting.
TriMark® Biopsy Site Markers
Optimal for thin-breasted patients and superficial lesions. Smooth marker deployment to the biopsy site with rigid end deploy bevelled tip.
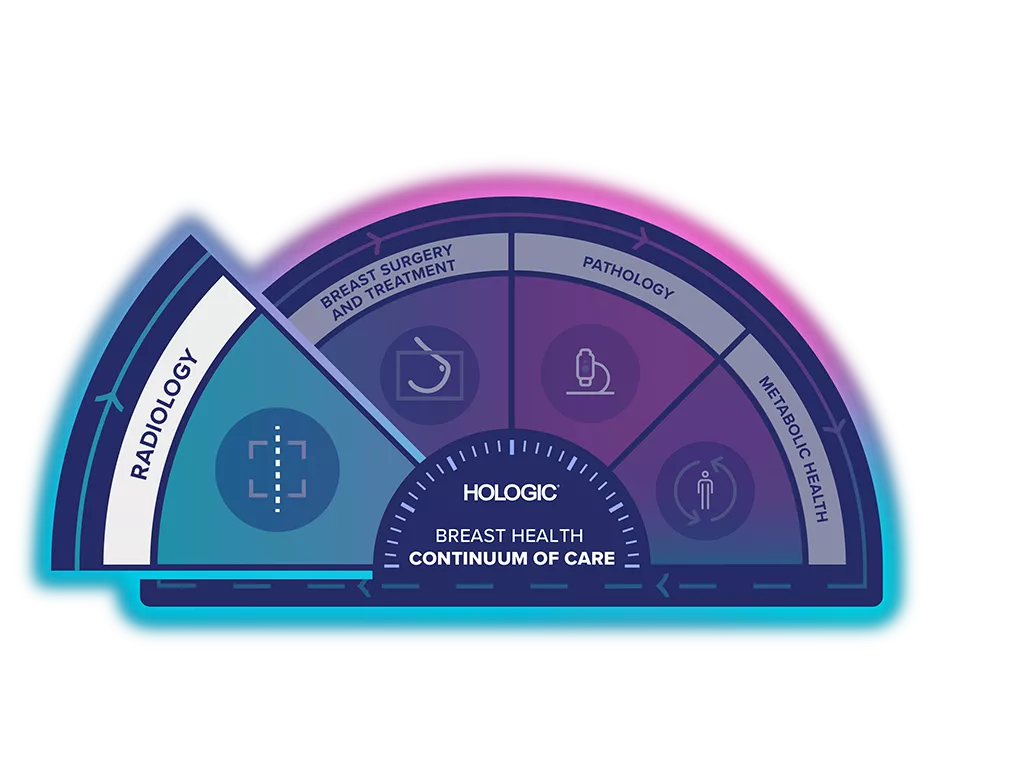
Unlock the Advantage of Time
The Breast Health Continuum of Care offers integrated solutions for clinical confidence, workflow efficiency and compassionate patient care. Giving more women, more time in better health.
The Breast Biopsy Site Marker range is part of the Hologic Localisation and Marking Solution.
Identify with Confidence
Research shows that the Tumark Professional X, Q and Vision biopsy site markers are accurate and easy to use.1
85%
say ultrasound visibility was good to excellent upon deployment1
92%
of markers deployed accurately to the intended area1
99%
agreed the device was easy to use1
Visit Our Virtual Hospital
Browse our portfolio of Breast Health solutions in 3D. See how you can unlock the advantage of time across the entire Breast Continuum of Care.
Browse the Comprehensive Range of Biopsy Site Markers
Image Gallery
Evidence. Insight. Collaboration.
Our education portal improves patient care through excellence in education, communication of clinical and scientific evidence, and partnerships with the healthcare community.
Insights
- Hologic Data on File. DHM-06169, Rev 001. * Based on Tumark® Data Collection Study, 3 clinicians at 3 hospitals for 90 marker placements, 2017.
- Yen P, Dumas S, Albert A, et al. Post-Vacuum-Assisted Stereotactic Core Biopsy Clip Displacement: A Comparison Between Commercially Available Clips and Surgical Clip. Can Assoc Radiol J. 2018 Feb;69(1):10-15. doi: 10.1016/j.carj.2017.08.004. PMID: 29458952. Devices show reduced displacement when compared to traditional metal surgical clips (28% and 27% vs 38% displacement, p = 0.001 and p = 0.0001). Visibility is dependent on surrounding tissue, experience may vary.
- Pinkney DM, Shah BA. Prospective Comparative Study to Evaluate the Sonographic Visibility of Five Commercially Available Breast Biopsy Markers. Journal of Diagnostic Medical Sonography. 2013;29(4):151-158. doi:10.1177/8756479313486962. *45.4 % of respondence rated SecurMark high sonographic visibility after 6-weeks post procedure, 4x more than the Gel Mark UltraCor™
- Hologic Data on File. MISC-07876, Rev 001, attachment 2. Rüland 2018 (n=50), Kuemmel 2020 (n=300) PMCF Hologic survey (n=29).
- Hologic Data on File. MISC-07876, Rev 001, attachment 2. Appendix G, Customer Feedback Survey.
- Hologic Data on File. MISC-07876, Rev 001, attachment 2. Flores-Funes 2021 (n=60), Jain 2017 (n=9), Siegmann 2009 (n=29), Wienbeck 2017 (n=3), Stahl 2021 (n=114), Rüland 2018 (n=50), Woodard 2019 (n=1).
- Hologic Data on File. MISC-07876, Rev 001, attachment 2 , Attachment 45. Flores-Funes 2021 (n=60), Hyde 2019 (n=50), Siegmann 2009 (n=29), Wienbeck 2017.
- Hologic Data on File. MISC-07876, Rev 001, attachment 2. Flores-Funes 2021 (n=60), Jain 2017 (n=9), Siegmann 2009 (Woodard 2019 (n=1)n=29), Wienbeck 2017 (n=3), Stahl 2021 (n=49).
Safety Data Sheets
Package Inserts
Related Products
2797
0482
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable
EC Representative Information wherever applicable