Definitive Diagnosis Starts with HPV mRNA


Know Your mRNA From Your DNA
A specific and sensitive HPV mRNA test may help improve efficiencies across cervical screening programmes in the UK, easing pressure from the labs to colposcopy teams and reducing the burden on patients.1

Improving Diagnosis, Efficiency, and Patient Experience in HPV Screening
Introducing mRNA HPV testing into screening pathways may help to promote overall efficiencies, ultimately leading to a better outcome for the NHS and women. One thing that is key to all of this is a definitive HPV diagnosis.1
mRNA HPV Assay: Cost-Saving Cervical Screening
Learn more about how using an mRNA HPV assay compared to a DNA HPV assay in cervical screening programmes could yield cost savings and reduce unnecessary testing and procedures.1-4
Benefits of HPV mRNA Testing
Improve efficiencies
Many women are waiting far longer than they should for a referral after reporting an abnormal HPV result.5 To mitigate this, HPV primary screening must run as efficiently and effectively as possible so that the right women are seen at the right time.6
Improve diagnosis
Within screening, mRNA testing allows for greater specificity due to detecting actively transcribing high-risk HPV mRNA, as opposed to transient infections, while remaining as sensitive as DNA counterpart tests.1
Improve patient experience
Introducing HPV mRNA testing means putting your patient first, by reducing the impact on the patient receiving false positive results, unnecessary colposcopy procedures, overtreatment, and psychosocial burden.1,7
Better Outcomes for the NHS and for Women
Introducing mRNA HPV testing could save the NHS £15.4 million, while reducing the number of unnecessary colposcopies by 29%.1 To learn more about the benefits of HPV mRNA testing, read our article.
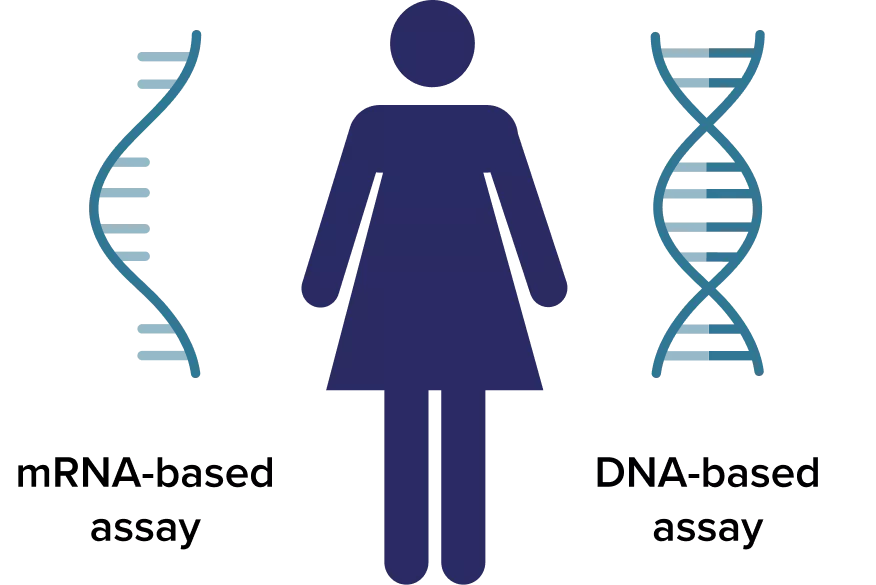
HPV Cervical Screening Outcome: mRNA vs DNA
Using the Aptima® HPV mRNA based assay, instead of a HPV DNA based test, can result in:*
- A 3.7% reduction in unnecessary HPV tests performed1
- A 42.6% reduction in unnecessary cytology tests performed1
- Up to a 24% reduction in false positives compared to HPV DNA test8
- Use of the Aptima HPV mRNA testing reduces colposcopy referral rates by 21% compared to Hybrid Capture 2*** (DNA), which may reduce the burden on the NHS and for women.**9-11
To learn more about the Aptima HPV mRNA based assay, click the button below:
Widening the Scope of Cervical Cancer Screening
Despite effective early treatment of cervical cancer, European screening rates are as low as 25%.12
There is an opportunity to increase participations rates when adopting the WHO's 90-70-90 strategy of vaccination, screening and treatment to work towards the elimination of cervical cancer.13 To learn more about how to optimise strategies to increase screening participation rate locally, read the ACCESS Consensus Group Report.

Hologic is a Leader in Pap and Human Papillomavirus (HPV) Testing
The Aptima HPV assay identifies high-risk HPV mRNA that is indicative of the HPV infections most likely to lead to cervical disease.14
As your trusted partner for an end-to-end diagnostic solution for cervical cancer screening, our solutions help you to improve your colposcopy service, boost efficiency, provide definitive testing, and reduce the burden on your patients.
Please contact us for more information on mRNA-based HPV testing and its benefits.
Information on data processing can be found in our privacy policy.
Insights
*Based on a probabilities HPV+ for year 1: DNA – 0.2026, mRNA – 0.1232; year 2: DNA (for women with normal reflex cytology in year 1): DNA – 0.6486, mRNA – 0.5505; and year 3 (for women with normal reflex cytology in year 2): DNA – 0.4283, mRNA – 0.3153.
**Based on statistical calculation. DNA, deoxyribonucleic acid; HPV, human papillomavirus; M, million; mRNA, messenger ribonucleic acid; NHS, National Health Service
***HC2 is a product from Legal Manufacturer Qiagen. Hologic is not responsible for the performance of this product. For further information, please refer to Qiagen : https://www.qiagen.com/us/products/diagnostics-and-clinical-research/sexual-reproductive-health/cervical-cancer-screening/digene-hc2-hpv-dna-test
- Weston, G. et al. (2020) Use of the APTIMA mrna high-risk human papillomavirus (HR-HPV) assay compared to a DNA hr-HPV assay in the English cervical screening programme: A decision tree model based economic evaluation, BMJ Open. Available at: https://bmjopen.bmj.com/content/10/3/e031303 (Accessed: 29 August 2023).
- Ibáñez R, Mareque M, Granados R, Andía D, García-Rojo M, Quílez JC, Oyagüez I. Comparative cost analysis of cervical cancer screening programme based on molecular detection of HPV in Spain. BMC Womens Health. 2021 Apr 26;21(1):178. doi: 10.1186/ s12905-021-01310-8.
- Weston, et al. A health economic model to estimate the costs and benefits of an mRNA vs DNA high-risk HPV assay in a hypothetical HPV primary screening algorithm in Ontario, Canada. Prev Med Rep. 2021 Jun 10;23:101448. doi: 10.1016/j.pmedr.2021.101448.
- Dombrowski CA, Weston GM, Descamps PP, Izopet PJ, Adams EJ, Adams E. Health economic evaluation of an mRNA high-risk human papillomavirus (HR-HPV) assay versus a DNA HR-HPV assay for the proposed French cervical screening programme. Medicine (Baltimore). 2022 Jul 22;101(29):e29530. doi: 10.1097/MD.0000000000029530.
- Jo’s Cervical Cancer Trust [internet] Behind the headlines: Colposcopy delays in Greater Glasgow and Clyde [cited 2023 July 20] Available from: https://www.jostrust.org.uk/about-us/news-and-blog/blog/behind-headlines-colposcopy-delays-greater-glasgow-and-clyde
- Rebolj M, Rimmer J, Denton K et al. Primary cervical screening with high risk human papillomavirus testing: observational study. 2019;364:l240
- McBride E, Marlow LAV, Forster AS et al. Anxiety and distress following receipt of results from routine HPV primary testing in cervical screening: The psychological impact of primary screening (PIPS) study. Int J Cancer. 2020;146(8):2113-21.
- Strang T H.R, Gottschlich A, Cook D A, Smith L W. Long-term cervical precancer outcomes after a negative DNA- or RNA-based human papillomavirus result. Am J Obstet Gynecol. 2021 November; 225(5):511.e1–511.e7. doi:10.1016/j.ajog.2021.05.038. Page 16. Table 3. (2021)
- Iftner T, Haedicke J. A review of the clinical performance of the Aptima HPV assay J Clin Virology. 2016
- Sawaya GF, Kuppermann M. Identifying a "range of reasonable options" for cervical cancer screening. Obstet Gynecol. 2015;125(2):308-10.
- Sauter L, Mount SL - Testing of integrated HPV mRNA decreases colposcopy referrals: could a change in human papillomavirus detection methodology lead to more cost-effective patient care? 2014;58(2):162-6. doi: 10.1159/000358246. Epub 2014 Feb 12.
- OCED. Health at a Glance. Europe 2018. Available at: https://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-europe-2018_health_glance_eur-2018-en
- World Health Organization. Global strategy to accelerate the emimilation of cervical cancer as a public health problem 2020. Available at: https://www.who.int/publications-detail-redirect/9789240014107
- Aptima HPV assay [package insert]. AW-14517-001, Rev. 001 (EN). San Diego, CA: Hologic, Inc.; 2016.
2797
Hologic BV, Da Vincilaan 5, 1930 Zaventem, Belgium.
Notified Body number wherever applicable
EC Representative Information wherever applicable
WEB-01956-GBR-2101 Rev 001 © 2024 Hologic, Inc. All rights reserved. Hologic and associated logos are trademarks and/or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries. All other trademarks are the property of their respective owners. This information is intended for medical professionals and is not intended as a product solicitation or promotion where such activities are prohibited. Because Hologic materials are distributed through websites, podcasts and tradeshows, it is not always possible to control where such materials appear. For specific information on what products are available for sale in a particular country, please contact your Hologic representative or write to [email protected].